Peter Gray
02 August 2021
Air Best Practices
Since the beginning of the COVID pandemic, it has become very apparent just how dangerous viruses and bacteria can be. Awareness has unfortunately grown of airborne bacteria transported in the ambient air.
Airborne bacteria can also be present in a compressed air system. The difference being the concentration is significantly higher in compressed air compared to ambient air. In most cases the concentration is 7 times as severe, as when the system pressure is 102 psig (7 barg).

Removal of bacteria from compressed air often overlooked
When compressed air comes into direct contact with a product, many applications believe their “standard” particulate and coalescing oil-removing filters, installed either side of a compressed air dryer, are sufficient to protect the downstream processes. Strangely enough, the removal of bacteria is often overlooked, despite this level of filtration being readily available, easy to procure, install and maintain.
We must protect against the contamination of bacteria, as it will typically destroy any product it comes into contact with. The benefits of removing bacteria from compressed air (and make it sterile), wherever compressed air comes (directly or indirectly) into contact with any type of food, beverage, or pharma product, are to increase shelf life, reduce product spoilage, negate the risk of recalls and bad press, all while increasing the quality and integrity of the end product.
Let us take as examples dairy production, food process, brewery, pharmaceutical or beverage manufacturing facility. Most of these processes, direct inject, blanket/purge tanks, open or fill packaging, or move their product using compressed air. Many do so without realizing that with the addition of a sterile filter, we elevate the compressed air quality to an absolute retention rate of 99.99998% related to 0.2 micron. This eradicates bacteria and microorganism contamination from the downstream compressed air flow.
Ensuring 0.2 micron sterile filtration
The conditions, within a compressed air system, are ideal environments for the growth of bacteria when the compressed air carries water vapour. Compressed air at higher temperatures carrying water vapor provides an environment in which bacteria are able to grow and reproduce quickly. In order to protect and preserve products from being contaminated with bacteria it is critical to provide the filtration able to produce sterile air.
In most factories we visit, this simply means all they require is the installation of one sterile filter assembly. It is critical to ensure the filter element rating is 0.2 micron to guarantee the bacteria do not penetrate the filter. Otherwise the filter element would not ensure sterility. The 0.2 micron rating is required and sized accordingly because it is the most difficult particle size to capture. Sterile filters are specifically designed to remove microorganisms and bacteria which are on average 0.2 micron.
Because the removal of moisture from compressed air can sometimes be inconsistent, it is recommended to protect processes by placing the sterile filter assembly as close to the direct compressed air contact with product/packaging as possible.
We recommend compressed air is dried, before it reaches the sterile filter assembly, because the moisture has a derogatory effect on the sterile element. If we remove the moisture to a pressure dew point of -40°F (-40°C) through the use typically of a desiccant air dryer, we are sufficiently removing water vapor to prevent bacteria and virus growth. It is always essential to dry and prefilter the compressed before the compressed air enters the sterile filter.

Stainless steel housings for sterile filters
The sterile filter assembly is made up of two components, the housing and the filter element.
The housing is constructed of stainless steel, with 304 and 316L SS being the most common off the shelf offering. The housing must be stainless steel to make certain it will not corrode. Corrosion is created by oxidation and the generation of unwanted particles, some of which provide food for the bacteria to feed on. The surface of the stainless steel housing is highly polished, as a rough surface of an unpolished stainless steel housing creates hiding places, in which the bacteria continue to grow.
Special order/custom options are also available, such as electro-polished outer surfaces on housings and all manner of inlet and outlet connection options. Connection options can include ASA (weld), DIN/ANSI, NPT, Tri-clamp ASME, Dairy Union DIN 11851, Flange EN1092-1 and Weld End.

Sterile filter elements and media
High quality sterile filter elements are available with FDA and EC validation and end users must insist the cartridges be shipped in a sealed and sterile package to ensure the filtration integrity and validation is guaranteed at every level. High quality elements are biologically and chemically inert, ensuring there is zero breeding ground for bacteria or separated microorganisms to reproduce. Consumers should also ensure the elements they are purchasing/using are 100% guaranteed non-shedding.
The filter media itself is also very important. High quality sterile filter element media will be binder-free. The fibers are thermally welded (sintered) together and with that are totally chemically inert offering no feeding ground for bacteria.
All other common types of filter elements use binders, glues and additives to hold the fibers together. This has two disadvantages:
- When the filter elements are sterilized, the glue becomes soft allowing movement of the fibers, which can cause them to become unsterile.
- Glues offer a feeding ground for bacteria.
This is not the case in a sterile filter element, which is manufactured using only a binder-free filter media.

Steam sterilization “regenerates” sterile filter elements
Once Bacteria is trapped by the sterile filter, we must exterminate the bacteria and prevent it from migrating beyond and growing through the filter element. It is recommended to sterilize a sterile filter after every production run - or at least after 14 days.
Extended product shelf life, and the peace of mind that sterile filtration provides, is further enhanced by the fact that the sterile filter elements can be “regenerated” between 100 and 200 times, depending on the grade of the sterile filter element.
Sterile filters can be “sterilized in-place” (SIP), with steam. Alternatively, the element can be removed from the housing and externally sterilized by autoclave. If a spare filter element is available, the elements can be rotated between the autoclave and the housing. Replacing the element with a new one is another option if steam or autoclave are unavailable.
The Steam sterilization temperature will need to be between 230°F (110°C) and 284°F (140°C), subject to the availability and temperature of steam. The sterilization times are between 30 minutes (at 230°F) to 10 minutes (at 284°F).
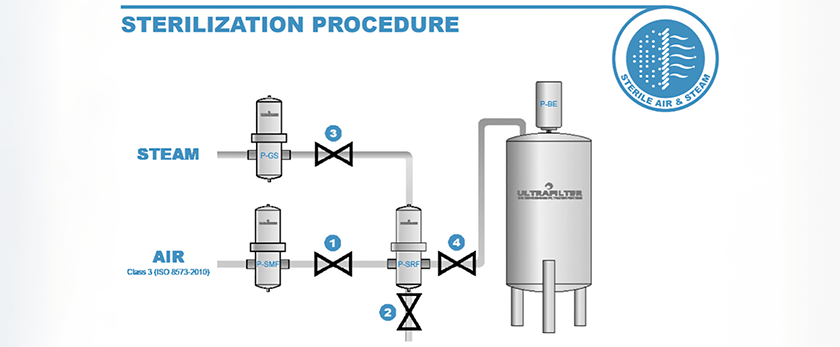
The sterilization process is simplistic and highly effective.
Step 1. Valve (1) and valve (4) close, to isolate the sterile housing and element.
Step 2. Drain valve (2) opens, to exhaust compressed air pressure.
Step 3. Valve (3) opens to allow steam to flow into the sterile filter housing.
Step 4. After reaching a temperature of 212°F(100°C), the steam begins to condense, with valve (2) remaining open. Steam pressure increases to the desired sterilization temperature.
Step 5. After reaching the required steam temperature, the actual sterilization process begins, using the following temperature and timeline ratios:
- Saturated steam 250°F (121°C) = 30 minutes
- Saturated steam 268°F (131°C) = 20 minutes
- Saturated steam 286°F (141°C) - 10 minutes
When the sterilization time has passed, valve (3) can be closed. Partially open valve (1) to introduce compressed air into the housing and purge any residual steam condensate out of the drain valve (2). Next close valve (2) and slowly open valves (1) and (4) fully.
Preventive maintenance ensures sterile filtration
Ensuring preventive maintenance is adhered to is a critical part of ensuring compressed air remains sterile at all times. From our experience, it goes well beyond replacing filter elements on time. One must create a maintenance plan outlining:
- The location of the filter on the plant floor
- The date the element was installed and when it should be replaced
- The frequency of steam sterilization, autoclave or element replacement
- Location of element inventory, onsite stores or vendor-controlled stores.


