Do you work in dairy or beverage production, brewing, food processing, or the pharmaceutical industry?
Introducing a Simple, Cost Effective Way to Improve Your End Product.
One thing that production facilities, shown above, have in common is that they all use compressed air in a variety of processes (i.e., direct injection, blanket/purge tanks, open or fill packaging, and product movement). In each of those processes, compressed air comes into direct contact with the product. Although most of these applications require the air to meet the ISO 8573.1 quality standard, the removal of bacteria is often overlooked, despite the fact that this extra level of filtration is readily available and easy to procure, install, and achieve.
When you consider that sterilized compressed air can increase shelf life, reduce product spoilage, increase the quality and integrity of the end product, and significantly reduce the risk of recalls, it's worth taking a closer look at the options. The fact is that each of these industries stands to benefit significantly from the supply of sterile compressed air.

Sterile Filtration Elevates Your Compressed Air Quality
At Air Solutions Canada, we're proud to represent Ultrafilter's line of filtration housings and elements. Ultrafilter is one of the most innovative manufacturers of high-performance filters and treatment components for compressed air, technical gases and liquids.
In most production facilities, a standard two-stage compressed air filter system (with a pre and after filter installed on either side of an air dryer) is used to produce a nominal particulate retention of 0.1 micron and oil carryover of 0.01 ppm. But point-of-use applications are best protected when the sterile filter assembly is placed as close as possible to where the direct compressed air contacts the product or packaging.
The addition of Ultrafilter sterile filtration elevates the compressed air quality to an absolute retention rate of 99.99998 % related to 0.2 micron. This eradicates bacteria and microorganism contamination from the downstream compressed air flow. In most factories we visit, this means that all they require is the installation of a single high quality sterile filter assembly.
The Ultrafilter P-SRF Sterile Filter Assembly
Ultrafilter's P-SRF sterile filter assembly is made up of two components: housings and elements.

1. P-EG Ultrafilter housing
The P-EG Ultrafilter housing is constructed of stainless steel, with 304 and 316L SS being the most common off-the-shelf offering. Special order/custom options are also available, such as electropolished surface finishes, and all manner of inlet and outlet connection options.

2. P-SRF Ultrafilter Elements
The P-SRF Ultrafilter elements are FDA and EC validated and shipped in a sealed and sterile package ensuring that they are ready to use, with guaranteed filtration integrity. Because Ultrafilter elements are biologically and chemically inert, there is zero breeding ground for bacteria or separated microorganisms to reproduce. All Ultrafilter sterile elements are 100-per-cent guaranteed and non shedding.
Extending the Life of Your Sterile Filters
Extended product shelf life and the peace of mind that Ultrafilter sterile filtration provides to an end user are further enhanced by the fact that all Ultrafilter sterile filter elements can be regenerated between 100 and 200 times, depending on the type of element purchased.
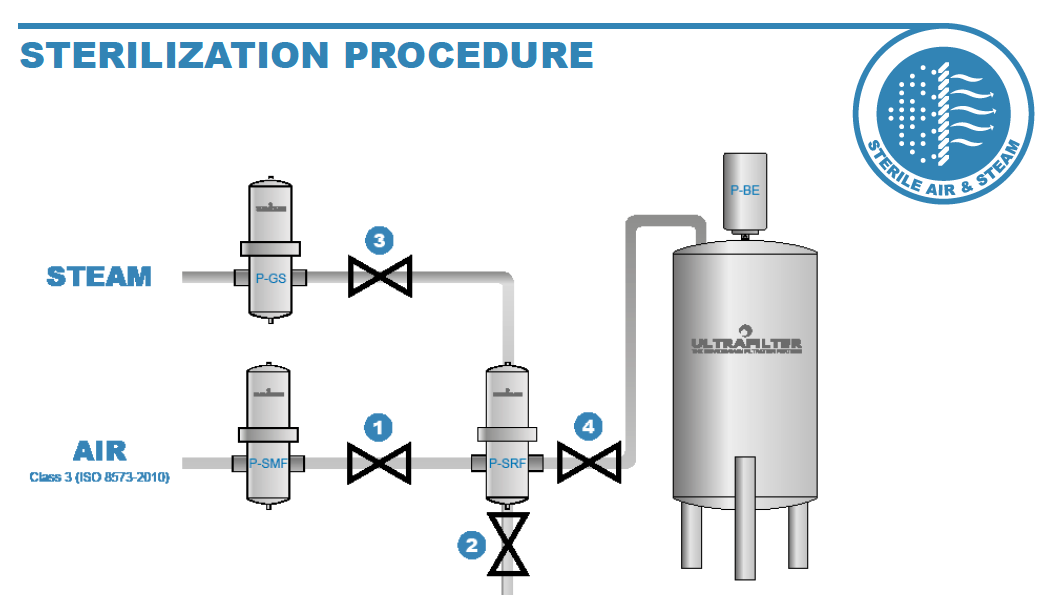
Ultrafilter sterile filters can be sterilized in place (SIP) using steam (the process is outlined below). Alternatively, if steam is not available, the element can be removed from its housing and externally sterilized by autoclave (by keeping a spare filter element on hand, the elements can be rotated between the autoclave and the housing, so production can continue without delays).
A sterile filter should be sterilized after every production run, or at least after 14 days of use. The steam sterilization temperature must be between 110°C and 140°C with the sterilization times being between 10 and 30 minutes, subject to steam temperature.
While sterilizing your filters helps to reduce costs and waste, depending on the quality assurance standards you wish to achieve in your industry, you may also choose to replace your filters with new ones every 14 production days.
5 Easy Steps to Sterilize a P-SRF Sterile Filter Element
The sterilization process is simple and highly effective and Ultrafilter offers a full range of clean steam and sanitary steam housings and elements, all of which are available for purchase through Air Solutions Canada.